Definition:-
It is thermodynamically unstable system consisting of at least two immiscible liquid phases one of which is dispersed as globules (the dispersed phase) in the other liquid phase (the continuous phase) stabilized by presence of emulsifying agent.
An emulsion consists of two immiscible liquids one of which is uniformly dispersed through the other as droplets of diameter greater than 0.1 micron m.
The system is stabilized by the presence of emulsifying agent. The particle diameter of the disperse phase extends from 0.1 to 100 micron m.
The system is stabilized by the presence of emulsifying agent. The particle diameter of the disperse phase extends from 0.1 to 100 micron m.
Types of emulsions:
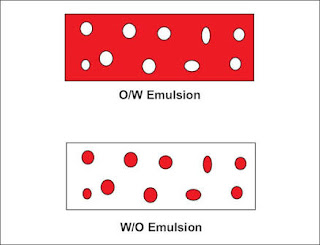
1) Oil in water (o/w) :-
i. Oil droplets are dispersed in a continuous aqueous phase.
ii. This emulsion is generally formed if the aqueous phase constitutes more than 45 % of the total weight and a hydrophilic emulsifier is used.
iii. These are [referred for oral administration and cosmetics.
iv. These are useful as water washable drug bases.
v. The globule size is 0.25 to 10 microns.
i. Oil droplets are dispersed in a continuous aqueous phase.
ii. This emulsion is generally formed if the aqueous phase constitutes more than 45 % of the total weight and a hydrophilic emulsifier is used.
iii. These are [referred for oral administration and cosmetics.
iv. These are useful as water washable drug bases.
v. The globule size is 0.25 to 10 microns.

2) Water in oil (w/o):-
i. Aqueous droplets are dispersed in continuous oily phase.
ii. This emulsion is generally formed if the oily phase constitutes more than 45 % of the total weight and a lipophilic emulsifier is used.
iii. These are used for cosmetics.
iv. They are employed for treatment of dry skin and emollient applications.
i. Aqueous droplets are dispersed in continuous oily phase.
ii. This emulsion is generally formed if the oily phase constitutes more than 45 % of the total weight and a lipophilic emulsifier is used.
iii. These are used for cosmetics.
iv. They are employed for treatment of dry skin and emollient applications.
O/W and W/O type emulsion
1) In this type, oil is in dispersed phase & water is in continuous phase.
--In this type of emulsion water is in dispersed phase & oil is in continuous phase.
2) These type of emulsion are preferred for internal use
--Mainly used externally as lotions or creams.
--Mainly used externally as lotions or creams.
3) Emulsifying agents are used gum acacia, tragacanth,methyl cellulose, saponins, synthetic subs & soaps from monovalent bases like Na+ , K+, NH4+
--Wool fat, resins, bees wax, & soaps from divalent bases like Ca++
4) Dilution Test:- Emulsion diluted with water result:- Emulsion remains stable
--Result:- Emulsion breaks on its dilution with water
5) Dye Test:- Emulsion scarlet red dye result:- Dispersed globules appear ‘red’ & ground is “Colourless”
--Result :- Disperse globules appear “Colourless” & ground is “red”
6) Conductivity Test: - This type of emulsion shows bulb glowing on passing the electric current.
--Bulb doesn’t glow because oil is in continuse phase
7) Fluorescence Test: - droplets shows fluorescence when U.V. rays passes.
2) Dye Solubility Test:-
--Wool fat, resins, bees wax, & soaps from divalent bases like Ca++
4) Dilution Test:- Emulsion diluted with water result:- Emulsion remains stable
--Result:- Emulsion breaks on its dilution with water
5) Dye Test:- Emulsion scarlet red dye result:- Dispersed globules appear ‘red’ & ground is “Colourless”
--Result :- Disperse globules appear “Colourless” & ground is “red”
6) Conductivity Test: - This type of emulsion shows bulb glowing on passing the electric current.
--Bulb doesn’t glow because oil is in continuse phase
7) Fluorescence Test: - droplets shows fluorescence when U.V. rays passes.
Test for identification of emulsion type:-
1. Dilution test (miscibility test)
2. Staining test (dye solubility test)
3. Conductivity measurement
4. CoCl2 filter test
5. Fluorescence test
2. Staining test (dye solubility test)
3. Conductivity measurement
4. CoCl2 filter test
5. Fluorescence test
1) Dilution test (miscibility test):-
An emulsion can only be diluted with its continuous phase. .
O/w can be diluted with water and w/o can be diluted with oil. So when oil is added to o/w emulsion or water is added to o/w emulsion, separation of dispersed and continuous phase occurs. This test is useful for liquid emulsions.
O/w can be diluted with water and w/o can be diluted with oil. So when oil is added to o/w emulsion or water is added to o/w emulsion, separation of dispersed and continuous phase occurs. This test is useful for liquid emulsions.
2) Dye Solubility Test:-
In this test, when an emulsion is mixed with a water soluble dye such as amaranth and observed under the microscope, if the continuous phase appears red, then it means that the emulsion is o/w type as water is the external phase and the dye will dissolve in it to give color but if the scattered globules appear red and continuous phase colorless, then it is w/o type. Similarly if an oil soluble dye such as Scarlet red C or Sudan III is added to an emulsion and the continuous phase appears red, then it w/o emulsion.
3) Conductivity Test:-
Water is good conductor of electricity whereas oil is non-conductor. Therefore, continuous phase of water runs electricity more than continuous phase of oil.
4) CoCl2 filter test: -
Filter paper impregnated with CoCl2 and dried ( blue) changes to pink when o/w emulsion is added. It may fail if emulsion is unstable or breaks in presence of electrolyte.
5) Fluorescence test: -
Since some oils fluoresce under UV light, 0/w emulsions exhibit dot pattern, w/o emulsion fluoresce throughout. But this test is applicable if oil has the property of fluorescence.