- After administration a drug has to fight its way for absorption however, various factors affect the rate of absorption they are listed as follows,
- Physico-chemical, Physiological and Pharmaceutical.
A. PHYSICOCHEMICAL FACTORS:
(ii) Particle size and effective surface area
(iii) Polymorphism and amorphism
(iv) Pseudo polymorphism (hydrates / solvates)
(v) Salt form of the drug
(vi) Lipophilicity of the drug – (pH partition hypothesis)
(vii) pKa of the drug and pH – (pH partition hypothesis)
(viii)Drug stability
B. PHYSIOLOGICAL FACTORS
- These includes factors relating to the anatomic, physiologic and pathologic characteristics of the patient.
(ii) Gastric emptying time
(iii) Intestinal transit time
(iv) Gastrointestinal pH
(v) Disease states
(vi) Blood flow through the GIT
(vii) Gastrointestinal contents:
a) Other drugs b) Food c) Fluids d) Other normal GI contents
(viii) Pre-systemic metabolism by
a) Luminal enzymes b) Gut wall enzymes c) Bacterial enzymes d) Hepatic enzymes
C. PHARMACEUTICAL FACTORS:
(i) Disintegration time (tablets / capsules)(ii) Dissolution time
(iii) Manufacturing variables
(iv) Pharmaceutical ingredients (excipients / adjuvants)
(v) Nature and type of dosage form
(vi) Product age and storage conditions
1. Drug solubility and dissolution rate:
- Orally administered solid dosage form are first disintegrated or disaggregated, then the solid particles are dissolved; drugs in solution then permeate across bio membrane to be absorbed in the body.
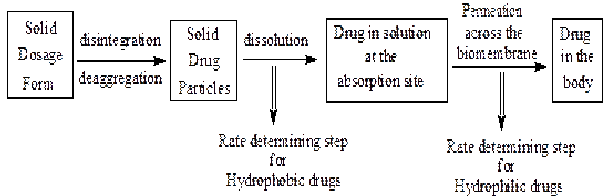
- Two critical processes in the absorption of orally administered drugs are:
2. Rate of drug permeation through the bio membrane (i.e. gastrointestinal membrane)
- · For poorly water-soluble drugs rate of dissolution is the rate determining step hence the absorption is called to be dissolution rate limited. e.g. Griseofulvin, Spironolactone.
- · For highly water-soluble drugs dissolution is rapid so the rate determining step is permeation hence, the absorption is called to be permeation rate limited. e.g., Cromlyon sodium, Neomycin sulfate etc.
2. Particle size and effective surface area of the drug particles.
- From Noyes-Whitney’s equation of dissolution:
![clip_image002[232] clip_image002[232]](https://blogger.googleusercontent.com/img/b/R29vZ2xl/AVvXsEgX7lg0skRAHHiutdeLBbSdChNTbrqel2it7p3XfSXZDuLkK6hBlqvvmyvGIVYWf0EYd0n82xzDQOB2cVAc2xwr0fZCPOPBJwrrODVVwlC1vOCtAQQhRCcbU2MzDQhgRIzKvUyH_rDGQwE/?imgmax=800)
where,
D = diffusion coefficient or diffusivity of the drug molecule
A = surface area of the dissolving solid exposed to the dissolution medium
Kw/o = water/oil partition coefficient of the drug
V = volume of dissolution medium
h = thickness of the stagnant layer
Cs – CB = concentration gradient of the diffusing drug molecule.
- From this equation it can be concluded that the greater the surface area, A, faster the distribution.
- When the particle size of a certain mass of a drug is reduced the surface area is increased, hence, if particle size is reduced dissolution rate increases.
- Two types of surface area can be defined:
2. Effective surface area: Which is the area of solid surface exposed to the dissolution medium.
- e.g. Micronization of poorly water soluble drugs like Griseofulvin, Chloramphenicol and several salts of Tetracycline results in superior dissolution rates.
- However, size reduction has some limitations.
- In case of hydrophobic drugs like Aspirin, Phenacetin and Phenobarbital micronization actually results in a decrease in effective surface area due to the following reasons.
(ii) The particle reaggregate to form larger particles due to their high surface free energy.
(iii) Extreme particle size reduction may impart surface charges that may prevent wetting; moreover electrically induced agglomeration may prevent intimate contact of the drug with the dissolution medium.
3. Polymorphism and amorphism:
- Depending on the internal structure, a solid can exist either in a crystalline or amorphous form.
- · When, a substance exists in more than one crystalline form, the different forms are designated as polymorphs and the phenomenon as polymorphism.
- N.B. Various polymorphs can be prepared by crystallizing the drug from different solvents under diverse conditions.
- Depending on their relative stability, one of the several polymorphic forms will be physically more stable than the others.
- Such a stable polymorph represents the lowest energy state, has highest melting point and least aqueous solubility.
- The remaining polymorphs are called metastable forms which represents higher energy state, the metastable forms have a thermodynamic tendency to convert to the stable form.
- A metastable form cannot be called unstable because if it is kept dry, it will remain stable for years.
- ·So the metastable forms have higher aqueous solubility and hence higher bioavailability than the stable polymorphs.
- e.g. Chloramphenicol palmitate has three polymorphs A, B and C. The B -form shows best bioavailability and A form is virtually inactive biologically.
- e.g. Polymorphic form-III of Riboflavin is 20 times more water soluble than the form-I.
- Due to aging of dosage forms containing metastable forms of the drug results in the formation of less soluble, stable polymorph.
- e.g. more soluble crystalline form-III of Cortisone acetate converts to less soluble form-V in an aqueous suspension resulting in caking of solid.
Amorphous form (i.e. having no internal structure)
- Such drugs represents the highest energy state and can be considered as super cooled liquids.
- They have greater aqueous solubility than their crystalline form. (Less energy taken while dissolving than crystalline form as they lack definite structure.)
- e.g. the amorphous form of the Novobiocin is 10 times more soluble than the crystalline form.
- Thus the order for dissolution of different solid forms of drug is amorphous > metastable > stable.
4. Pseudo polymorphism (Hydrates / Solvates):
- During crystallization process the solvent molecules may be incorporated into the crystal lattice of the solid in stoichiometric proportion – these type of crystals are called solvates; and the trapped solvent molecules as solvent of crystallization.
- The solvates again can remain in different polymorphic states, called as pseudopolymorphs. The phenomenon is called as pesudopolymorphism.
- When the solvent with the drug is water, the solvate is known as hydrate.
Effect on absorption:
- · Generally, the anhydrous form of a drug has greater solubility than the hydrates.
- This is because the hydrates are already in equilibrium with water and therefore have less demand for water.
- e.g. anhydrous form of Theophylline and Ampicillin have higher aqueous solubility, dissolve at faster rate and show better bioavailability in comparison to their monohydrates and trihydrate forms respectively.
- · On the other hand nonaqueous solvates have greater aqueous solubility than the nonsolvates.
- e.g. n-pentanol solvate of Fludrocortisone and Succinyl Sulfathiazole and the chloroform solvates of Griseofulvin are more water soluble than their non-solvate forms.
5. Salt form of the drug:
- Most drugs are either weak acids or weak bases. One of the easiest approach to enhance the solubility and dissolution rate of such drugs is to convert them into their salt forms.
- Weak acid HA is more soluble in basic pH and weak base B is more soluble in acidic pH by the formation of salt.
- Some time in-situ salt formation can be utilized, e.g. certain drugs like aspirin and penicillin are prepared as buffered alkaline tablets.
- When the tablets are put into water the pH of the microenvironment of the drug is increased which promotes the dissolution rate.
- So buffered aspirin tablets have two advantages
(ii) in dry form the hydrolytic stability is better.
(iii) bioavailability is increased by increasing the dissolution.
·
Size of counter ion
- Smaller the size of the counter ion (of the salt form of a drug) greater the solubility of the salt. e.g. bioavailability of Novobiocin from its sodium salt, calcium salt and free acid forms are in the following ratio:
50 20 1
Ionic strength of the Counter ion:
- When the counter ion is very large in size and/or has poor ionic strength (as in the case of ester form of the drugs), the solubility may be much lower than the free drug itself.
- prolong the duration of action – e.g. steroidal salts
- overcome bad taste – e.g. Chloramphenicol
- overcome GI-instability – e.g. erythromycin estolate
- decrease the side effects, local or systemic.
6. pKa of the drug and pH:
Drug pKa and lipophilicity and GI pH (pH partition theory)- The pH partition theory (Brodie et.al.) states that for drug compounds of molecular weight greater than 100, which are primarily transported across the biomembrane by passive diffusion.
- The process of absorption is governed by,
2. lipid solubility of the unionized drug (Ko/w)
3. the pH at the absorption site
- The above statement of the hypothesis was based on the assumptions that:
2. Larger the fraction of unionized drug, faster the absorption.
3. Greater the lipophilicity (Ko/w) of the unionized drug, better the absorption.
Handerson-Hasselbach equation:
- The amount of drug that exists in unionized form is a function of dissociation constant (pKa) of the drug and pH of the fluid at the absorption site.
for weak acid: |
|
 |
|
|
Drugs
|
pKa
|
pH at the site of absorption |
| Very weak bases Theophylline Caffeine Oxazepam Diazepam |
(pKa < 5.0) 0.7 0.8 1.7 3.7 |
Unionized at all pH values: absorbed along the entire length of GIT. |
| Moderately weak bases Reserpine Heroin Codeine Amitriptyline |
(5 < pKa < 11) 6.6 7.8 8.2 9.4 |
Ionized at gastric pH, relatively unionized at intestinal pH better absorbed from intestine. |
| Stronger base Mecamylamine Guanethidine |
(pKa > 11.0) 11.2 11.7 |
Ionized at all pH values: poorly absorbed form GIT. |
- It is the pKa of the drug that determines the degree of ionization at a particular pH and that only the unionized drug, if sufficiently lipid soluble, is absorbed into the systemic circulation.
- Ideally, for optimum absorption, a drug should have sufficient aqueous solubility to dissolve in the fluids at the absorption site and lipid solubility (Ko/w) in the lipoidal biomembrane and into the systemic circulation.
- In other words, a perfect hydrophilic-lipophilic balance (HLB) should be there in the structure of the drug for optimum bioavailability.
B. PHYSIOLOGICAL FACTORS
I. Physiology of GIT
- The major components of the GIT are stomach, small intestine (duodenum, jejunum and ileum) and large intestine (colon) which differ from each other in terms of anatomy, function, secretions and pH.
- The mean length of the entire GIT is 450 cm.
- The entire inner surface of GIT from stomach to large intestine is lined by a thin layer of muco-polysaccharides (mucous membrane) which normally acts as a barrier to bacteria, cells or food particles.
- The gastrointestinal tract shows a wide variety of pH which offers different areas for absorption of different drugs as follows,
| 1. Mouth | pH 6 – 8 | small surface area | lipophilic, neutral and basic drugs are absorbed directly |
| 2. Stomach | pH 1 – 3 | not too large a surface area | lipophilic, neutral and acidic drugs absorbed but lesser than that from intestine |
| 3. Small intestine | pH 5 – 7.5 | very large surface area | major site for absorption of all types of drugs (lipophilic, neutral, acidic or basic) |
| 4. Large intestine | pH7.9–8.0 | small surface area | all types of drugs are absorbed but to a lesser extent |
| 5. Rectum | pH 7.5–8.0 | much smaller surface area | all types of drugs are absorbed, about half of the absorbed drug goes directly into the systemic circulation and the other half to the liver |
Stomach
- The stomach is a bag like structure having a smooth mucosa and thus small surface area. Its acidic pH, due to its secretion of HCl, favors absorption of weakly acidic drugs like aspirin.
Small intestine

Fig. Components of intestinal epithelium
- The folds in intestinal mucosa, called as fold of Kerckring result in 3 fold increase in surface area.
- The surface of this folds possess finger like projections called as villi which increases the surface area by 30 times.
- From the surface of villi protrude several microvilli resulting in 600 times increase in the surface area. All these combined to impart a large surface area of more than 200 sq.m.
- The blood flow is 6 – 10 times more than that of in stomach.
- pH range is 5 to 7.5 which is more favorable for most drugs to remain unionized.
- The peristaltic movement of intestine is slow, transit time is long, and penetrability is high. All this factors make intestine the best site for absorption of most drugs.
Large intestine
- Its length and mucosal surface area is very small (villi and microvilli are absent) compared to small intestine and thus absorption of drug from this region is very small.
- However, because of the long residence time (6 to 12 hrs.), colonic transit may be important in the absorption of some poorly soluble drugs and sustained release dosage forms.
II. Patient related factors
1. Age
In infants,- gastric: pH is high
- intestinal surface is small
- blood flow is less.
- altered gastric emptying
- decreased intestinal surface area
- decreased GI blood flow
- achlorhydria
- bacterial overgrowth in small intestine.
2. Gastric emptying
- Passage of gastric content from stomach to small intestine is called gastric emptying.
- Rapid gastric emptying is required where:
(ii) dissolution of drug occurs in the intestine e.g. enteric coated dosage forms.
(iii) the drugs are not stable in gastric fluid e.g. Penicillin-G and Erythromycin.
(iv) the drug is best absorbed from the distal part of the small intestine e.g. vitamin B12.
- Delay in gastric emptying is required where:
(ii) disintegration and dissolution of dosage form is promoted by gastric fluid
(iii) the drugs are absorbed from the proximal part of the small intestine e.g. vitamin B2 and vitamin C.
· Gastric emptying is a first order rate process. Several parameters are used to quantify gastric emptying:
(i) Gastric emptying rate is the rate at which the stomach content empty into the intestine.
(ii) Gastric emptying time is the time required for the gastric content to empty completely into the small intestine.
(iii) Gastric emptying t1/2 is the time taken for half the stomach contents to empty.
- N.B. In vivo gastric emptying can be studied by using radio-opaque contrast materials (e.g. BaSO4) or tagging the drug with a radio-isotope and scanning the stomach at regular intervals of time.
Factors influencing gastric emptying rate:-
1. Volume of meal:- Larger the volume of meal longer the gastric emptying time.
- The rate of gastric emptying for various food materials is in the following order:
carbohydrates > protein > fats
3. Physical state and viscosity of meal- Liquid meals take less than an hour to empty solid meals take as long as 6 – 7 hours to empty.
- Viscous material empty at a slow rate in comparison to less viscous materials.
- High or low temperature of the ingested fluid (compared to body temperature) reduce gastric emptying rate.
- Gastric emptying is retarded at low stomach pH and is promoted at higher or alkaline pH.
- Water, isotonic, and solutions of low salt concentration empty the stomach rapidly whereas higher electrolyte concentration decreases gastric emptying rate.
- Gastric emptying is favored while standing and while lying on the right side; while lying on the left side or in supine position retards it.
- Stress and anxiety promote gastric motility whereas depression retards it.
- Vigorous physical exercise retards gastric emptying.
- Diseases like gastroenteritis, gastric ulcer, pyloric stenosis, diabetes and hypothyroidism retard gastric emptying.
- Drugs that retard gastric emptying includes
(ii) Anticholinergics e.g. Atropine, Propantheline
(iii) Narcotic analgesics e.g. Morphine
(iv) Tricyclic antidepressants e.g. Imipramine, Amitriptyline.
- Drug that stimulate gastric emptying are:
(ii) Domperidone (Domstal)
(iii) Cisapride (Alpride)
3. Effect of GI pH on drug absorption:
- GI fluid pH influence drug absorption in several ways:
1. Disintegration
- The disintegration of some dosage forms is pH sensitive. With enteric coated formulations, the coat dissolves only in the intestinal pH, followed by disintegration of the tablet.
2. Dissolution
- A large number of drugs are either weakly acidic or weakly basic whose solubility is greatly affected by pH. A pH that favours the formation of salt of the drug enhances the dissolution rate. e.g. Weakly acidic drugs dissolve rapidly in the alkaline pH of the intestine whereas basic drugs dissolves in the acidic pH of the stomach.
3. Absorption
- Depending upon the pKa of the drug and the pH of the GI fluid some amount of the drug remain in ionized state and some in unionized state.
- The unionized form will be absorbed through GIT quickly than the ionized form.
4. Stability
- GI pH influences the chemical stability of drugs. e.g. The acidic stomach pH is known to affect degradation of Penicillin-G and Erythromycin.
4. Effect of GI content
- A number of GI contents can influence drug absorption.
1. Food-drug interaction
- Presence of food may either delay, reduce, increase or may not affect drug absorption.
| Delayed | Decreased | Increased | Unaffected |
| Aspirin Paracetamol Diclofenac |
Penicillins Erythromycin Ethanol Tetracycline Levodopa, Iron |
Griseofulvin Diazepam |
Methyldopa Sulphasomidine |
- As a general rule, drugs are better absorbed under fasting conditions and presence of food retards or prevents it.
- Food does not significantly influence absorption of a drug taken half an hour or more before meals and two hours or more after meals.
- Delayed or decrease drug absorption by food can be due to one or more of the following reasons:
(b) Preventing the transit of enteric tablets into the intestine which may be as long as 6 – 8 hrs.
(c) Formation of poorly soluble, unabsorbable complex e.g. Tetracycline-Calcium complex (Chelate).
(d) Increased viscosity due to food thereby preventing drug dissolution and/or diffusion towards the absorption site.
- Increased drug absorption following a meal can be due to the following reasons:
(b) Enhanced solubility due to GI secretions like bile.
(c) Prolonged residence time and absorption site contact of the drug e.g. water-soluble vitamins.
·
Types of meal
(i) Meals high in fat aid solubilisation of poorly aqueous soluble drugs like Griseofulvin.(ii) Food high in proteins increases oral availability of propranolol because
a) such a meal promotes blood flow to the GIT helping in drug absorption.
b) increases hepatic blood flow due to which the drug can bypass first-pass hepatic metabolism (Propranolol is a drug with high hepatic metabolism)
5. Drug-drug interaction
- Drug-drug interactions can be either physicochemical or physiological.
(a) Physicochemical drug-drug interactions can be due to –
Adsorption:
- Antidiarrheal preparations containing adsorbents like attapulgite or kaolin-pectin retard / inhibit absorption of Promazine and Lincomycin when co-administered with them.
Complexation:
- Antacids containing heavy metals such as Aluminium, Calcium, Iron, Magnesium or Zinc retard absorption of tetracycline due to the formation of unabsorbable complexes called as chelates.
pH change:
- Basic drugs dissolve in gastric pH. Co-administration of sodium bicarbonate with tetracycline results in evaluation of stomach pH and hence decreases dissolution rate or cause precipitation of drug.
(b) Physiologic drug-drug interaction can be due to following reasons:
Decreased GI transit:
- Anticholinergic drugs such as propantheline retard GI motility and promote absorption of drugs like ranitidine and digoxin.
Increased gastric emptying:
- Metoclopramide promotes GI motility and enhances absorption of Tetracycline, Pivampicillin and Levodopa.
Altered GI metabolism:
- Antibiotics inhibit bacterial metabolism of drugs e.g. Erythromycin enhances efficacy of digoxin by this mechanism.
6. Presystemic metabolism / First pass effects:
- The loss of drug through biotransformation by GIT and liver during the passage to systemic circulation in called First pass or presystemic metabolism.
- The 4 primary systems which affect presystemic metabolism of drugs are:
2. Gut wall enzymes /mucosal enzymes
3. Bacterial enzymes, and
4. Hepatic enzymes
1. Lumenal enzymes
- These are enzymes present in the gut fluids and include enzymes from intestinal and pancreatic secretions.
- Pancreatic enzymes contains hydrolases which hydrolyze ester drugs like chloramphenicol palmitate into active chloramphenicol.
- Peptidases split amide ( –CONH) linkages and inactivate protein / polypeptide drugs. Thus one of the approaches is to deliver them to colon which lacks peptidases.
2. Gut-wall enzymes (also called mucosal enzymes):
- They are present in stomach, intestine and colon.
- Stomach mucosa contains alcohol dehydrogenase (ADH) inactivates ethanol.
3. Bacterial enzymes:
- The GI microorganisms are scantily present in stomach and small intestine and is rich in colon. Hence, most orally administered drugs remain unaffected by them.
- The colonic microbes generally render a drug more active or toxic on biotransformation:
- e.g. Sulfasalazine (used in ulcerative colitis) is hydrolyzed to Sulfapyridine and 5-amino salicylic acid by the microbial enzymes of the colon.
- Digoxin, oral contraceptive drugs are absorbed in the upper intestine; exerted through bile as glucuronide conjugates.
- This conjugates of drugs are hydrolyzed by microbial enzymes.
- The free drugs are reabsorbed into the systemic circulation.
4. Hepatic enzymes:
- e.g. Isoprenaline, Propranolol, Alprenolol, Pentoxyfylline, Nitroglycerin, Diltiazem, Nifedipine, lidocaine, morphine etc.
C. PHARMACEUTICAL FACTORS
1. Disintegration time:
- Disintegration time (DT) is of particular importance in case of solid dosage forms like tablets and capsules.
- After disintegration of a solid dosage form into granules, the granules must deaggregate into finer particles and then dissolution takes place.
- If DT is long the bioavailability will be less.
- Rapid disintegration is thus important in the therapeutic success of a solid dosage form.
- DT increases with increase in the amount of binder and hardness of a tablet.
- Disintegration can be aided by incorporating disintegrants in suitable amounts during formulation.
2. Manufacturing / process variables:
- Dissolution from a solid dosage form depends on:
(A) excipients and (B) manufacturing process.
(A) Excipients:
- A drug is rarely administered in its original form.
- All dosage forms contains a number of suitable excipients (non-drug components of a formulation).
(a) Vehicle:
- Vehicle or solvent system that carries a drug is the major component of liquid orals and parenterals.
- The three categories of vehicles generally used are:
(ii) nonaqueous but water miscible e.g. propylene glycol, glycerol, sorbitol.
(iii) nonaqueous and water immiscible vehicle e.g. vegetable oils.
- Bioavailability of a drug from vehicle depends, to a large extent, on its miscibility with biological fluids.
- ·Aqueous and water miscible vehicles are rapidly miscible with body fluids (e.g. G.I.-fluid, tissue fluid, blood etc.) and drugs are rapidly absorbed from them.
- Propylene glycol, glycerol etc. are used as co-solvent to increase the solubility of a drug in water. Sometimes solubilisers, such as Tween 80 are used to promote solubility of a drug in aqueous vehicle.
- In case of water immiscible vehicles, the rate of drug absorption depends upon its partitioning from the oil phase to the aqueous body fluids, which could be a rate limiting step.
b) Diluents (Fillers):
- Diluents are commonly added to tablet (and capsules) formulations.
- Hydrophilic powders used as diluent are starch, lactose, microcrystalline cellulose etc. These hydrophilic powders forms a coating over the hydrophobic drugs particles (e.g. spironolactone and triamterene) and rendering them hydrophilic.
- Inorganic diluents like dicalcium phosphate (DCP) forms divalent calcium-tetracycline complex which is poorly soluble in water and thus unabsorbable.
c) Binders and granulating agents:
- These materials are used to hold powders together to form granules or promote cohesive compacts for directly compressible materials and ensure that the tablet remains intact after compression.
- Large amount of binders increase hardness and thus decrease disintegration / dissolution rates of tablets.
- Non-aqueous binders like ethyl cellulose also retard dissolution.
d) Disintegrants:
- These agents overcome the cohesive strength of tablet and break them up on contact with water.
- Almost all the disintegrants are hydrophilic in nature.
- A decrease in the amount of disintegrant can significantly lower the bioavailability.
e) Lubricants:
- These agents are added to tablet formulations to aid flow granules, to reduce interparticular friction and to reduce sticking or adhesion of particles to dies and punches.
- The commonly used lubricants are hydrophobic in nature (several metallic stearates and waxes). They reduce the wettability of particle surface, penetration of water into tablet.
- The best alternative is to use soluble lubricants like sodium lauryl sulphate and carbowax which promotes drug dissolution.
f) Suspending agents /Viscosity building agents:
- Agents like vegetable gums (acacia, tragacanth etc.), semisynthetic gums (carboxy methyl cellulose, methyl cellulose) and synthetic gums which reduces the sedimentation rate of a suspension
- The macromolecular gums often form unabsorbable complex with amphetamine.
- An increase in viscosity by these agents acts as a mechanical barrier to the diffusion of drug from the dosage form into the bulk of GI fluids.
h) Surfactants:
- Surfactants are widely used in formulations as wetting agents, solubilizers, emulsifiers, etc.
- Surfactants increase the absorption of a drug by the following ways:
2. Better membrane contact of the drug for absorption
3. Enhanced membrane permeability of the drug .
- Decreased absorption of drug in the presence of surfactants has been suggested to be due to :
2. Laxative action induced by a large surfactant concentration.
i) Complexing agents
- Several examples where complexation has been used to enhance drug bioavailability are:
- Enhanced dissolution through formation of a soluble complex
hydroquinone-digoxin complex.
- Enhanced lipophilicity for better membrane permeability e.g. caffeine-PABA complex (PABA = para amino benzoic acid) and
- Enhanced membrane permeability e.g. enhanced GI absorption (normally not absorbed from the GIT) in presence of EDTA (ethylene diamine tetraacetic acid) which chelates Ca++ and Mg++ ions of the membrane.
- Disadvantages of complexation:-
2. large molecular size of drug-protein cannot diffuse through the cell membrane.
j) Colorants
- Even a very low concentration of water-soluble dye can have an inhibitory effect on dissolution rate of several crystalline drugs.
- The dye molecules get adsorbed onto the crystal faces and inhibit drug dissolution – e.g. brilliant blue retards dissolution of Sulphathiazole.
- Dyes have also been found to inhibit micellar solubilisation effect of bile acids which may impair the absorption of hydrophobic drugs like steroids.
(B) Manufacturing process:
(i) method of granulation(ii) Compression force
(iii) Intensity of packing of capsules
i) Method of granulation:
- The wet granulation process is the most conventional technique of manufacturing tablet granules. The limitation of this method include –
(ii) the liquid may act as medium or affecting chemical reactions such as hydrolysis, and
(iii) the drying step may harm the thermolabile drugs.
- Wet granulation includes greater number of steps than dry granulation or direct compression which can adversely affect the dissolution.
ii) Compression force:
- The compression force employed in tableting process influence density, porosity, hardness, disintegration time and dissolution of tablets.
- The curve obtained by plotting compression force versus rate of dissolution can take one of the 4 possible shapes shown in the figures:

↑density and hardness of tablet
¯ ↓porosity, hence penetrability of the solvent into the tablet
¯ ↓wettability by forming a firmer and more effective sealing layer by the lubricant
B. Higher compression force
- causes deformation, crushing or fracture of drug particles into smaller ones or, convert a spherical granules into a disc shaped particle with large increase in effective surface area
- ↑ in dissolution rate
In short, the influence of compression force on the dissolution is difficult to predict.
(iii) Intensity of packing of capsule contents
Packing density in case of capsule can either inhibit or promote dissolution.
· Diffusion of GI fluids into the tightly filled capsules creates a high pressure within the capsule results in rapid bursting and dissolution of contents.
· In some cases capsules with tight packing
® pore size of the compact mass is decreased
® poor penetrability of GI - fluid
® poor rate of drug release
NATURE AND TYPES OF DOSAGE FORM
Cause of events that occur following oral administration of various dosage forms:

As a general rule, the bioavailability of a drug from various dosage forms decreases in the following order:
Solution > Emulsions > Suspensions > Capsules > Tablets > Coated tablets > Enteric coated tablets > Sustained release tablets.
Thus, absorption of a drug from solution is fastest with least potential for bioavailability problems whereas absorption from sustained release product is lowest with greatest bioavailability.