Transfer of Drug Molecules Across the Biological Barriers.
- For systemic absorption, a drug must pass from the absorption site through one or more layers of cells to gain access to the general circulation.
- For absorption into the cells, a drug must traverse the cell membrane.
STRUCTURE OF CELL MEMBRANE
- Cell membrane surrounds the entire cells and acts as a boundary between the cell and interstitial fluid.
- Cell membrane acts as a selective barrier to the passage of molecules. Water, some small molecules, and lipid-soluble molecules pass through such membrane; whereas highly charged molecules and large molecules, such as proteins and protein-bound drugs, can not.
Structure
- Cell membranes are generally thin, approximately 70 to 100 Å in thickness.
- They are primarily composed of phospholipids in the form of a bilayer.
- Some carbohydrates and proteins are interspersed within this lipid bilayer.
Lipid bilayer or Unit membrane theory (Proposed by Davson & Danielli; 1952):
- According to this theory, the cell membrane is composed of two layers of phospholipids between two surface layers of proteins.
- The hydrophilic “head” groups of the phospholipids facing the protein layers and the hydrophobic “tail” groups of the phospholipids aligned towards the interior.

* This theory can explain:
The observations that lipid-soluble drugs tend to penetrate cell membranes more easily than polar molecules.
* This theory cannot explain:
The diffusion of water, small molecules such as urea, and certain charged ions through this lipid-bilayer.
Fluid mosaic model (Proposed by Singer & Nicolson 1972):

- According to this model, the cell membrane consists of globular proteins embedded in a dynamic fluid, lipid-bilayer matrix.
- Integral proteins are embedded in the lipid bilayer;
- The integral proteins provide a pathway for selective transfer of certain polar molecules and charged ion through the lipid membrane.
- Peripheral proteins are associated with the inner and outer surfaces of the membrane.
- In the inner surface the peripheral proteins are attached to the fatty acid chain and at the outer surface, they are attached to the integral proteins or to oligosaccharides.
- The carbohydrates consist of monosaccharides attached together in chains that are attached to proteins (forming glycoproteins) or to lipids (forming glycolipids).
- Carbohydrates are always on the exterior side and peripheral proteins are always on the cytoplasmic or inner surface.
Mechanisms of Drug Transfer:
- The principal mechanisms of transport of drug molecules across the cell membrane are:
1. Passive diffusion
2. Carrier-mediated transport
(a) Active transport
(b) Facilitated transport
3. Vesicular transport
(a) Pinocytosis
(b) Phagocytosis
4. Pore transport
5. Ion pair formation
1. PASSIVE TRANSPORT
- Passive diffusion is the process by which molecules spontaneously diffuse from a region of higher concentration to a region of lower concentration.
- This process is passive because no external energy is expended.
Characteristics of passive transport
1. Drug molecules move from a region of relatively high concentration to one of lower concentration.
2. The rate of transfer is proportional to the concentration gradient between the compartments involved in the transfer.
3. The transfer process achieves equilibrium when the concentration of the transferable species is equal on both sides of the membrane.
4. Drugs which are capable of existing in both charged and a non-charged form approach an equilibrium state primarily by transfer of the non-charged species across the membrane.
5. Greater the membrane/water partition coefficient of drug faster the absorption [since the membrane is lipoidal in nature, a lipophilic drug diffuses at a faster rate by solubilising in the lipid layer of the membrane]
Mathematical expression
- Passive diffusion is best expressed by Fick’s first law of diffusion which can be expressed mathematically:

Fick's First Law of Diffusion 1
Several factors influence the passive diffusion of the drug:
1. The degree of lipid solubility of the drug (Km/w)
- Highly lipid soluble drug has a large value of Km/w and hence has a higher rate of transport.
2. The surface area of the membrane (A)
- The duodenal area shows most rapid drug absorption than that of other places of the intestine because the duodenal area has villi and microvilli, which provide a large surface area.
- These villi are less abundant in other areas of the GIT.
3. Thickness of the membrane (h)
- Drugs usually diffuse very rapidly through the capillary cell membrane except through the cell membranes present in the capillaries of the brain.
- In the brain, the capillaries are densely lined with glial cells, so a drug diffuses slowly into the brain.
2. CARRIER MEDIATED TRANSPORT:
- Some polar molecules cross the membrane more readily than can be predicted from their concentration gradient and partition coefficient values.
- This suggests the presence of some specialized transport mechanisms without which many essential water-soluble nutrients like monosaccharides, amino acids and vitamins will be poorly absorbed.
- The mechanism is thought to involve a component of the membrane called as the carrier that binds reversibly or noncovalently with the solute molecules to be transported.
- This carrier-solute complex traverses across the membrane to the other side where it dissociates and discharges the solute molecule.
- The carrier then returns to its original site to complete the cycle by accepting a fresh molecule of solute.
- The carrier may be an enzyme or some other component of the membrane.
Characteristics of Carrier-Mediated Transport:
1. The transport is structure specific i.e. the carrier can bind with a specific chemical structure only.
- Since the system is structure-specific, drugs having structure similar to essential nutrients, called false-nutrients are absorbed by the same carrier system. e.g. 5-fluorouracil and 5-bromouracil serves as false nutrients.

2. As the number of carrier systems is limited there will be competition between similar chemical structures for the carrier molecules.
3. Since there are a finite number of carriers available, the system is capacity limited. If the total number of transferable molecules exceeds the number of carrier sites available for transfer, the system will become saturated. The system will then be working at full capacity and the transfer of drug may thus occur at a constant rate until the concentration of drug falls below that of the capacity limit of the system.
4. For a drug absorbed by passive diffusion the rate of absorption increases linearly with the concentration but in case of carrier-mediated process, the drug absorption increases linearly with concentration until the carriers become saturated after which it becomes curvilinear and approach a constant value at higher doses. Such a capacity limited process can be adequately described by mixed order kinetics also called as Michaelis-Menten saturation or non-linear kinetics.
- The process is called mixed order because it is first order at subsaturation drug concentration but apparent zero order at and above saturation levels.
N.B. The bioavailability of a drug absorbed by such a system decrease with increasing dose – for example vitamins like B1, B2 and B12. Hence administration of large dose of such vitamins is irrational.
5. Carrier-mediated absorption generally occurs from specific sites of the intestinal tract which are rich in a number of carriers. Such an area in which the carrier system is most dense is called an absorption window. Drugs absorbed through such absorption windows are poor candidates for controlled release formulations.
Active Transport
- The drug is transported from a region of lower concentration to a region of higher concentration, i.e. against the concentration gradient
1. Since the process is occurring against the concentration gradient hence, energy is required in the work done by the carrier.
2. As the process requires the expenditure of energy it can be inhibited by metabolic poisons that interfere with energy production like fluorides, cyanide and dinitrophenol and lack of oxygen.
3. It is a capacity limited process. When all the carriers become saturated the drug is carried at a constant rate.
- Endogenous substances that are transported actively include
Sodium (Na+), potassium (K+), calcium (Ca++), iron (Fe++) in ionic state;
certain amino acids and
vitamins like niacin, pyridoxine and ascorbic acid.
- Drugs having structural similarity to such agents are absorbed actively, particularly the agents used in cancer chemotherapy.
Examples: Absorption of 5-fluorouracil and 5-bromouracil via pyrimidine transport system,
Absorption of methyldopa and levodopa via L-amino acid transport system
Absorption of angiotensin-converting enzyme (ACE) inhibitor (e.g. enalapril)via the small peptide carrier system
Facilitated diffusion
- Facilitated diffusion is also a carrier-mediated transport system but it moves along a concentration gradient (i.e from higher to lower concentration) and hence it does not require any energy.
Characteristics:

· It is a carrier-mediated transport system.
· The carriers are saturable and structurally selective for a drug and shows competition kinetics for drugs having similar structures.
· It does not require any energy expenditure.
· Facilitated diffusion of ions takes place through proteins, or assemblies of proteins, embedded in the plasma membrane. These transmembrane proteins form a water-filled channel through which the ion can pass down its concentration gradient. The transmembrane channels that permit facilitated diffusion can be opened or closed. They are said to be "gated". Some types of gated ion channels:
- ligand-gated
- mechanically-gated
- voltage-gated
- light-gated
Examples:
· Acetylcholine (ligand) binds to a certain synaptic membrane and opens Na+ channels and initiate a nerve impulse.
· Gamma-aminobutyric acid (GABA) binds to GABAA receptors and the chloride channel opens. This inhibits the creation of a nerve impulse.
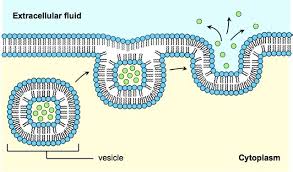
3. VESICULAR TRANSPORT:
- Vesicular transport is the process of engulfing particles or dissolved materials by the cell.
- There are two types of vesicular transport – Pinocytosis and Phagocytosis.
- Pinocytosis refers to the engulfment of small solutes or fluid. (Drinking by cell)
- Phagocytosis refers to the engulfment of larger particles or macromolecules, generally by macrophages. (Eating by cell.)
- Endocytosis and exocytosis are the processes of moving macromolecules into and out of a cell, respectively.
- During pinocytosis or phagocytosis, the cell membrane invaginates to surround the material and then engulfs the material, incorporating into the cell (fig). subsequently, the cell membrane containing the material forms a vesicle or vacuole within the cell.
e.g.
· Vesicular transport is the proposed process for the absorption of orally administered Sabin polio vaccine and large proteins.
· Transport of proteins, polypeptides like insulin from insulin-producing cells of the pancreas into the extracellular space.
4. PORE TRANSPORT:
- Very small molecules (such as urea, water, and sugars) are able to rapidly cross cell membranes as if the membrane contains channels or pores. [although pores are not evident microscopically].
- A certain type of protein called transport protein may form an open channel across the lipid membrane of the cell.
e.g.
· Drug permeation through aqueous pores is used to explain the renal excretion of drugs and the uptake of drugs into the liver.
5. ION PAIR FORMATION:

- Strong electrolyte drugs are highly ionized or charged molecules, such as quaternary nitrogen compounds with extreme pKa values.
- Strong electrolyte drugs maintain their charge at all physiologic pH values and penetrate the membrane very poorly.
- When ionized drugs are linked up with an oppositely charged ion, an ion pair is formed in which the overall charge of the pair is neutral. This neutral drug-complex diffuses more easily across the membrane.
e.g.
· Propranolol, a basic drug, forms an ion pair with oleic acid.
· Quinine forms an ion pair with hexyl salicylate.